A-1. Legislation overview
Section outline
-
-
Dr. Ioannis Dosis (German Environmental Agency) gives an overview of the regulatory landscape regarding chemical management, traceability and other related issues on EU level.
-
Alexandra Caterbow (HEJ Support) gives an overview of the regulatory landscape regarding chemical management, traceability and other related issues on global level.
-
-
Overview of REACH
The key elements of the REACH-Regulation (Regulation (EC) 1907/2006) are the registration, evaluation, authorisation and restriction of chemical substances. The Regulation is directly applicable in the Member States of the European Union since it entered into force on 1 June 2007.
REACH has the aim “to ensure a high level of protection of human health and the environment, including the promotion of alternative methods for assessment of hazards of substances, as well as the free circulation of substances on the internal market while enhancing competitiveness and innovation” (Article 1(1)).
The REACH Regulation is founded on the principle that manufacturers, importers, and downstream users are responsible for ensuring that the substances they produce, market, or use do not pose a risk to human health or the environment. This regulatory framework is guided by the precautionary principle.
Accordingly, the “no data, no market” rule applies, meaning that a substance can only be manufactured or placed on the market if the necessary data for its registration have been generated. Substances of Very High Concern (SVHCs) are to be gradually replaced with suitable alternatives or technologies, provided these are economically and technically feasible.
The implementation of the technical, scientific and administrative aspects of REACH is undertaken by the European Chemicals Agency (ECHA) in Helsinki.
REACH covers the manufacture, marketing and use of chemical substances whether they are used on their own (e.g., methanol or sodium hydroxide), in mixtures (e.g., paints), or incorporated into articles (e.g., textiles, tires, etc.).
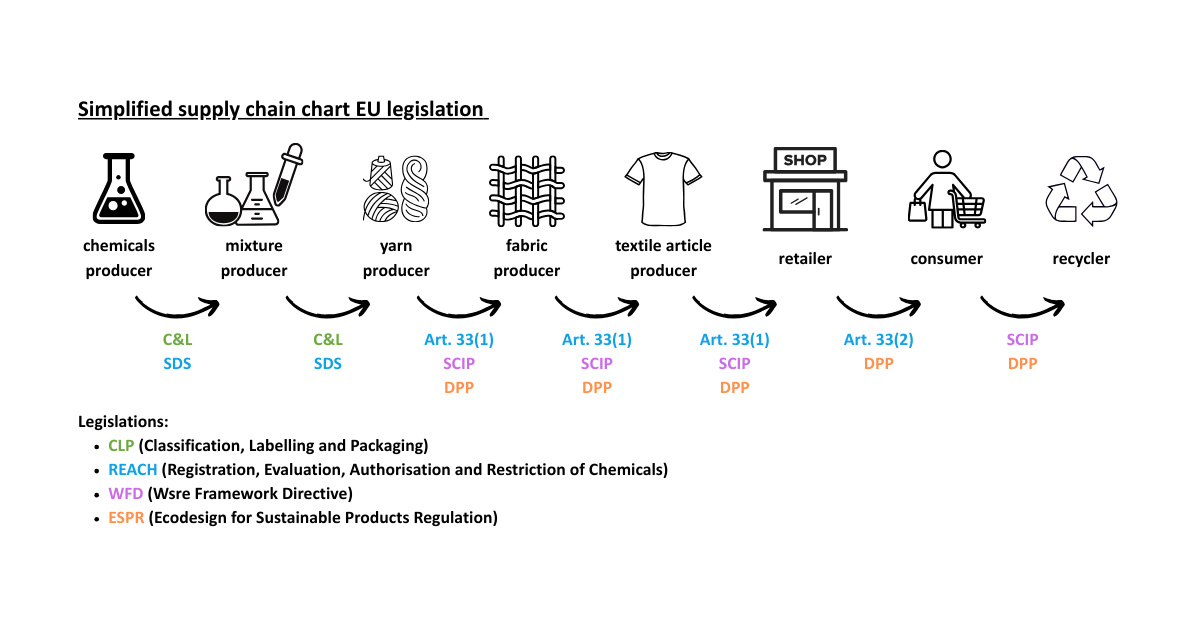
In this section, we will focus on the safety data sheet (SDS) and Article 33 of REACH that were mentioned in the simplified supply chain chart for EU legislation. Detailed guidance documents on the registration, evaluation, authorisation and restriction of chemical substances can be found on ECHA’s website.
Safety Data Sheet (SDS)
Safety Data Sheets (SDS) play a critical role in sharing information throughout the supply chain, including end users such as professional workers. They ensure that manufacturers and importers provide the necessary details to promote the safe use of substances and mixtures at every stage of their life cycle.
In addition to SDS, product labels serve as a key source of hazard information accessible to users, including both workers and consumers. Together, these tools help ensure that chemical risks are clearly communicated and understood.
A SDS includes comprehensive information such as the substance's properties, associated hazards, safe handling instructions, disposal methods and transport guidelines. It also outlines appropriate conditions for use and risk management measures to ensure both human health and the environment.
A SDS is mandatory (Article 31(1)) when:
• a substance or mixture fulfills the classification criteria as a dangerous product according to the CLP Regulation, or;
• a substance is persistent, bio-accumulative and toxic, or very persistent and very bio-accumulative, according to the criteria in Annex XIII REACH, or;
• a substance is included in the list of substances that are candidates for authorisation (SVHC) which could possibly be subjected to Authorisation in the future according to Article 59(1) REACH.A SDS is not required:
• in the case of articles
• for hazardous substances and mixtures made available to the general public (Article 31(4)), if the information for a safe use of the substances or a mixture is sufficient.A SDS must be supplied on request (Article 31(3)) for certain mixtures that are not classified but contain at least one substance:
• that presents a danger for health or the environment, in an individual concentration of ≥ 1 % by weight for non-gaseous mixtures and ≥ 0.2 % by volume for gaseous mixtures;
• being PBT (persistent, bioaccumulative and toxic), vPvB (very persistent, bioaccumulative and toxic), or SVHC in an individual concentration ≥ 0.1 % by weight for non-gaseous mixtures;
• having an EU workplace exposure limit.SDS are to be provided free of charge on paper, or in electronic format (Article 31(8)) and in the official language of the Member State in which the substance is being placed on the market (Article 31(5)). Information on the required languages of the SDS for each Member State is accessible in a document made available on ECHA’s website.
SDS must be updated (Article 31(9)):
• as soon as new information which can affect the management of risks or new information regarding hazards are made available;
• once an authorisation (under REACH) is granted or denied;
• as soon as a restriction (under REACH) has been adopted;
In addition, it is recommended to regularly review and update a SDS.The updated SDS is disseminated free of charge to all the former recipients for whom the providers have supplied the substance or mixture within the last 12 months.
Communication of information on substances in articles – Article 33
Article 33 aims to ensure that adequate information is communicated throughout the supply chain, enabling the safe use of articles by all end users, including consumers. This flow of information allows each actor in the supply chain to implement appropriate risk management measures at their stage of use, particularly when dealing with articles containing substances from the Candidate List. It also empowers both operators and consumers to make informed purchasing decisions.
According to Article 33(1) and 33(2), any supplier of an article must provide relevant safety information to the recipient of the article or, upon request, to a consumer when the following two conditions are met:
• the substance is included in the Candidate List for authorisation, and
• the substance is present in the article at a concentration above 0.1% weight by weight (w/w).Information must be provided to professional users and distributors the first time an article is supplied after a substance has been added to the Candidate List. For consumers, this information must be provided upon request, within 45 calendar days of the request and free of charge.
If no specific safety information is required to ensure the safe use of the article, for example, when exposure to the substance can be ruled out throughout all stages of the article’s lifecycle, including disposal, then at a minimum, the name of the substance must still be communicated. The information shared should clearly state that the substance is included in the latest version of the Candidate List and that this inclusion is the reason for the communication.
Regarding the obligations to communicate information on substances in articles (both to recipients and consumers), please note the following:
• The 0.1% w/w concentration threshold for Candidate List substances applies to every individual article supplied. This threshold applies to each article of an object made up of more than one article, which were joined or assembled together (complex objects).
• There is no tonnage threshold that triggers these communication obligations.
• A distributor supplying articles to consumers cannot fulfill their communication duty by simply referring the consumer to their own supplier or the article’s producer/importer.
• The obligation to communicate arises solely from the presence of a Candidate List substance in the article. This applies regardless of whether the supplier is aware of the substance’s presence. Therefore, suppliers are encouraged to proactively obtain information about Candidate List substances in their articles.
• The requirement to provide information upon a consumer’s request applies regardless of whether the consumer purchased the article in question. -
Overview of CLP
CLP (Regulation (EC) 1272/2008) is the acronym for Classification, Labelling and Packaging of substances and mixtures. This Regulation entered into force on the 20th of January 2009 and is based on the Globally Harmonized System (GHS), a set of recommendations drafted by the United Nations.
The purpose of the CLP regulation is to clearly communicate chemical hazards to workers and consumers across the European Union by classifying and labelling chemicals appropriately.
The primary goal of CLP is to protect human health and the environment. All parties involved in the supply chain are responsible for complying with the classification, labelling and packaging rules. Before chemical products enter the market, manufacturers and suppliers must assess the potential risks these substances and mixtures pose to people and the environment and classify them based on their identified hazards. Hazardous chemicals must be labelled using a standardised system to ensure that workers and consumers understand the risks before use.
Moreover, CLP is closely connected with:
• REACH, especially in relation to safety data sheets;
• various other European regulations, including those on Biocides, Plant Protection Products, major industrial accident prevention (Seveso Directive) and workplace chemical safety (Carcinogens and Mutagens Directive, Chemicals Agents Directive).CLP requirements apply to chemical substances (such as chloric acid, ethanol, iron, cooking salt, ammonia, bleach...) as well as to mixtures (detergents, paints, lacquers, concrete, oil...). Unlike the REACH Regulation, CLP does not provide any exemptions. Therefore, all hazardous chemicals falling under the scope of CLP must be notified and included in the Classification and Labelling (C&L) inventory.
In this section we will mainly focus on the C&L inventory. Visit ECHA’s webpage for further information and guidance on the CLP regulation.
Classification and labelling (C&L) inventory
The Classification and Labelling (C&L) inventory is a database maintained by ECHA that contains information on the classification and labelling of substances placed on the European market. It includes data on both notified and registered substances, as well as the list of harmonised classifications and labelling specified in Annex VI of the CLP Regulation.
Within the framework of the C&L inventory, manufacturers and importers are required to notify the classification of all substances placed on the market.
A notification to ECHA must be submitted when:
• substances are manufactured and/or imported and are subject to registration under the REACH Regulation;
• substances are classified as hazardous;
• mixtures contain a hazardous substance present above the relevant concentration limit, causing the mixture itself to be classified as hazardous;
• articles contain a substance that is subject to registration according to Article 7 of the REACH Regulation.The notification must be made within one month of placing the substance on the market. For importers, this one-month period begins from the date the substance, either on its own or within a mixture, is physically brought into the territory of the European Union.
Note:
• There are no exemptions from C&L notification requirements. Therefore, even substances exempt under the REACH Regulation must be notified.
• If a manufacturer or importer has already submitted a registration dossier under REACH for a substance, a separate C&L notification is not required since the necessary classification and labelling information is already included in the registration dossier.C&L notifications must be submitted electronically through the REACH-IT portal on the ECHA website. There are three submission methods available:
• Directly online using REACH-IT;
• Creating an IUCLID dossier and submitting the notification via REACH-IT;
• Uploading a bulk notification as an XML file through REACH-IT.More information and guidance concerning C&L notifications can be found on ECHA’s website.
-
The Ecodesign for Sustainable Product Regulation (ESPR) is an initiative, consisting of a package of measures. It entered into force in July 2024.
The ESPR provides concrete regulatory measures that help achieve the Green Deal’s circular economy goals, translating its broad goals into specific product requirements to ensure that the EU market shifts toward sustainable production and pushing manufacturers to adopt sustainable practices.
The new requirements will be defined under specific delegated acts for product groups, such as textiles, electronics or furniture. The delegated acts will detail the new requirements for the products, in terms of design, durability, reparability, recyclability, etc.
A new measure featured in the ESPR is the implementation of a Digital Product Passport (DPP). The DPP aims to electronically register, process and share information amongst the supply chain actors, authorities and consumers. It is defined by the European Commission as a structured collection of product related data with pre-defined scope and agreed data ownership and access rights conveyed through a unique identifier, and that is accessible via electronic means through a data carrier.
-
The Waste Framework Directive establishes key concepts and definitions for waste management, including what constitutes waste, recycling, and recovery. It also sets out fundamental principles for how waste should be managed. These include ensuring that waste is handled:
- without posing a threat to human health or the environment
- without endangering water, air, soil, plants, or animals
- without creating nuisances such as noise or unpleasant odours
- and without negatively impacting the countryside or protected areas of interest
The SCIP database, which stands for "Substances of Concern in articles as such or in complex objects (Products)" was established under the Waste Framework Directive to manage hazardous substances in products. Since 5 January 2021, companies placing articles on the EU market that contain substances of very high concern (SVHCs) from the Candidate List in concentrations above 0.1% weight by weight (w/w) are required to submit information about these articles to ECHA. This information is stored in the SCIP database, which ensures transparency about the presence of Candidate List substances throughout the entire lifecycle of products and materials—including at the waste stage. The database makes this information accessible to both waste operators and consumers.